C-Scan is the first and only preparation-free test to detect precancerous polyps and enable early intervention and cancer prevention.


More than one third of the U.S. screening age population avoids standard screening procedures such as colonoscopy. C-Scan has the potential to offer a more patient-friendly option, enabling people to get the screening they need.


How does C-Scan work?
Once the ingestible capsule C-Scan Cap is swallowed, it travels naturally along the gastrointestinal tract while scanning the inner lining of the colon. C-Scan Cap actively communicates with the second element of the system, C-Scan Track, which records and stores the scanning data. When the screening procedure is over, C-Scan Cap will be excreted naturally, the patient is notified, and the scanning data can be analyzed by using our proprietary software, C-Scan View.

C-Scan Cap
- Ingestible capsule
- Emission of ultra-low dose X-ray beams
- Scanning of gastrointestinal tract
- Autonomous and natural passage through the gastrointestinal tract
- Total X-ray exposure dose similar to that of a single chest radiography
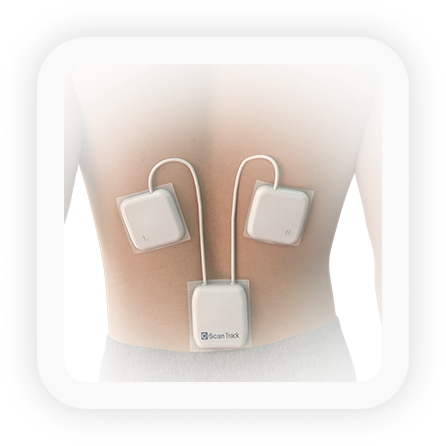
C-Scan Track
- Three miniaturized patches worn on the patient’s back
- Integrated positioning, control and recording system
- Continuous recording and storage of C-Scan Cap’s information

C-Scan View
- Cloud based analysis suite allows physicians to analyze data anywhere
- Construction of maps of the inner surface of the colon
- Pre-analysis and bookmarking of suspicious findings by an expert technician


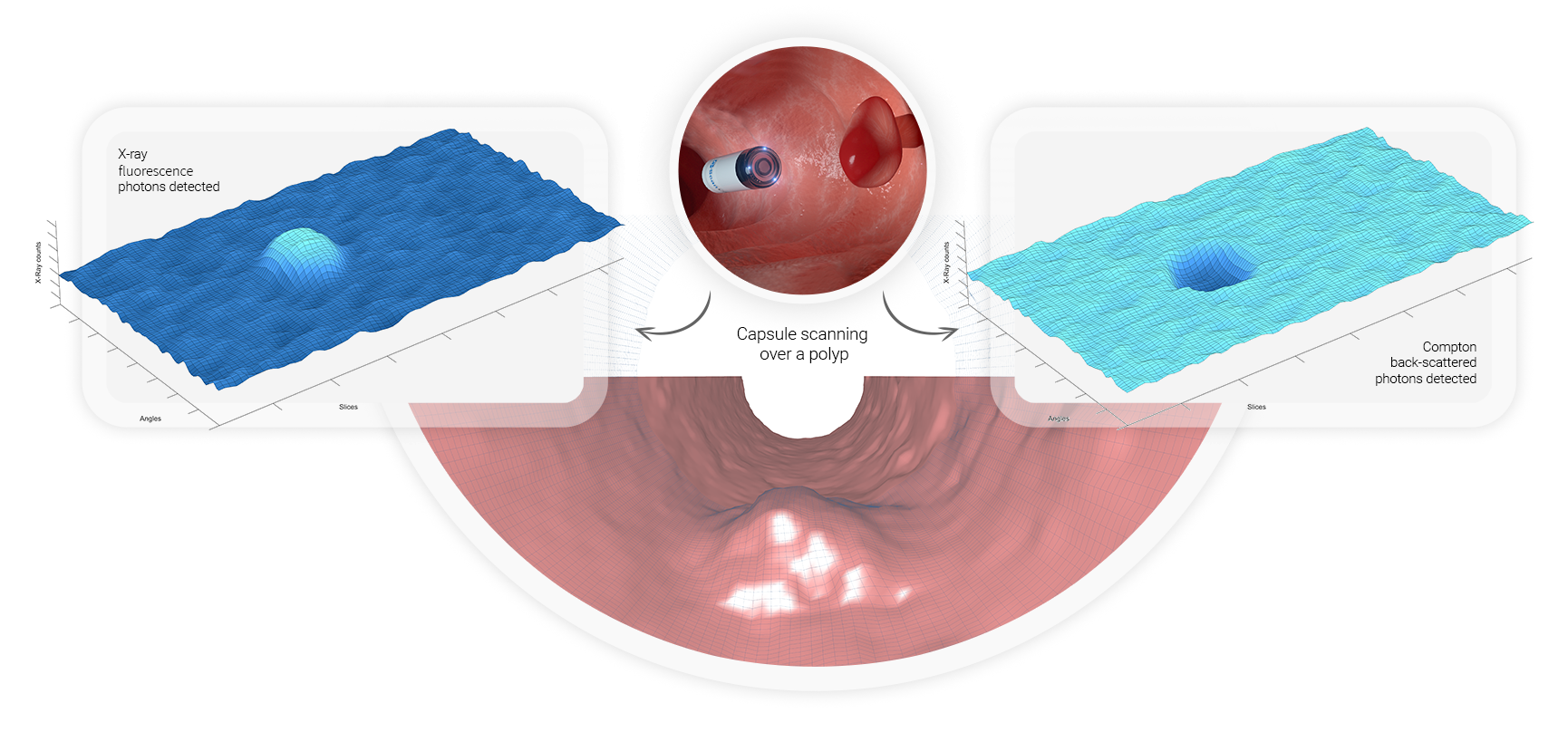
The C-Scan’s technology is based on two physical phenomena related to the interaction of the X-ray photons emitted by the capsule with the contents of the colon and the tissue wall.
The number of detected X-ray fluorescence photons and Compton back-scattered photons is correlated to the distance between the capsule and the colon wall. As the distance between the capsule and the colon wall increases, presence of content induces higher rates of X-ray fluorescence photons. At the same time, the content attenuates Compton back-scattered photons, hence decreasing the corresponding detection rate.
The close proximity of the capsule to the scanning target allows for the use of an ultra-low dose radiation, since a very low x-ray flux is required to obtain sufficient signal-to-noise ratio and good scanning quality.
CLINICAL DATA
Clinical Study Results For C-Scan in Israel and the EU
In a multi-center, open label prospective study performed in Israel, the C-Scan System demonstrated high sensitivity and specificity for the detection of precancerous polyps when compared to FIT (fecal immunochemical test), a commonly used non-invasive colorectal cancer screening test. Colonoscopy was used as the reference method.
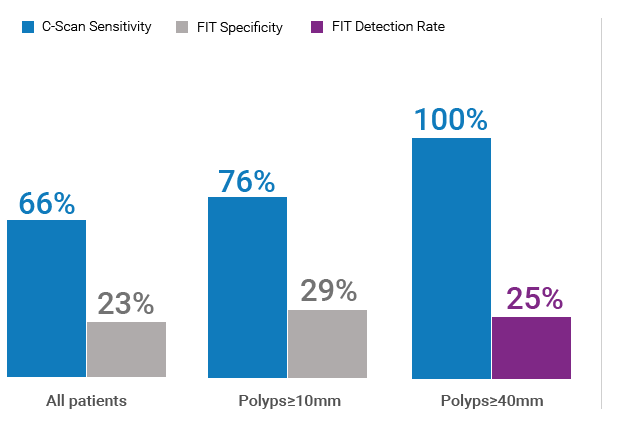
- Sensitivity – ability to correctly identify patients with polyps
- Specificity – ability to correctly identify patients with lack of polyps
Sensitivity

Specificity


U.S. Pilot Trial Results
Check-Cap completed a pilot study in the U.S. to evaluate the safety, usability and subject compliance of the C-Scan® system. The study was conducted at two sites, the New York University Grossman School of Medicine and Mayo Clinic.
- Positive results were reported from 28 evaluable study subjects (out of 40 who completed the study).
- The primary endpoint was achieved, and no device or procedure related serious adverse events were reported.
- All patients who underwent the study complied with the procedure and completed a questionnaire after the test, reporting higher satisfaction with the C-Scan System compared to colonoscopy.
- The study also showed an agreement between C-Scan and colonoscopy in detection of polyps for evaluable patients, consistent with data from the post-CE approval study*.
*Due to sample size, the study was not designed to be powered for statistical significance
Regulatory approvals
The C-Scan® system has received CE mark in Europe and approval from the Israeli Ministry of Health, the Medical Device Division (AMAR), for marketing in Europe and Israel, respectivelly.
Publications & Presentations/Events
Potential screening benefit of a colorectal imaging capsule that does not require bowel preparation
A novel prepless X-ray imaging capsule for colon cancer screening
Radiographic capsule-based system for non-cathartic colorectal cancer screening
Reconstruction method for x-ray imaging capsule
Phantom system for intraluminal x-ray imaging of the human colon
An x-ray based capsule for colorectal cancer screening incorporating single photon counting technology
Approach to Incomplete Colonoscopy: New Techniques and Technologies
Novel prep-less X-ray imaging capsule for colon cancer screening: a feasibility study
The C-Scan System is not approved in the United States and is currently an investigational device there.
The system is approved in the E.U. and Israel but it is not available for sale in any jurisdiction.
This website is not intended to provide medical advice for patients or healthcare providers.